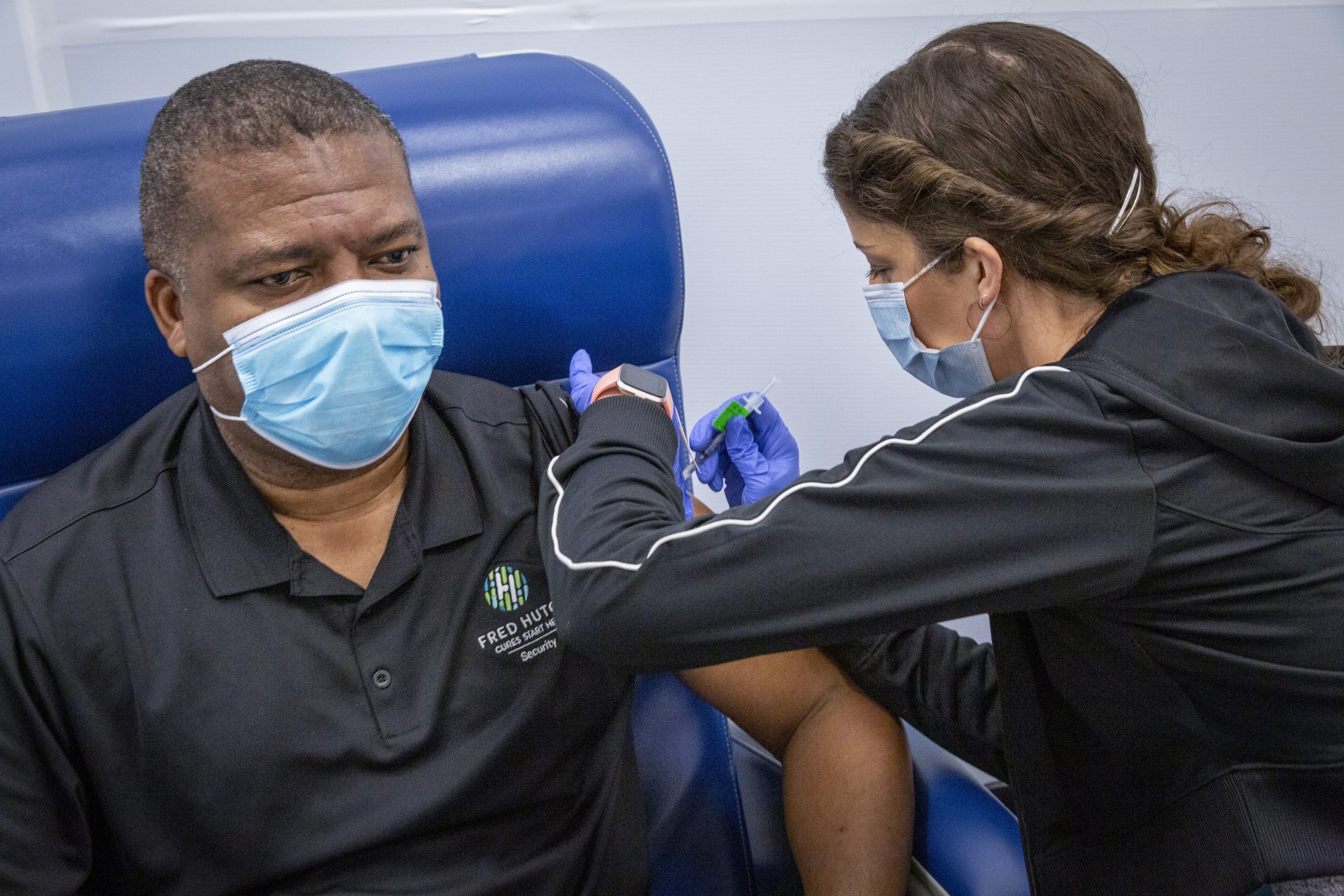
Anthony Jackson, Safety Coordinator for the Fred Hutchinson Cancer Research Center, received a dose of the Pfizer-BioNTech COVID-19 vaccine on January 19 at a new vaccination clinic on the Fred Hutch campus. (Courtesy photo by Fred Hutch / Robert Hood)
With data showing racial disparities in the distribution of COVID-19 vaccines, a new study found racial and ethnic inequalities in nearly a decade of US vaccine trials.
Minority communities and older adults were underrepresented in relation to their overall share in the population. That was the result of a study of more than 200 vaccine studies published on the JAMA Network Open on Friday.
The results are in line with a new Washington state report that shows a lower percentage of Hispanic, black, and multiracial people receiving COVID-19 vaccines compared to their overall proportion of the state’s population.
Nationwide, color communities also carried a disproportionate burden during the pandemic with higher rates of hospital stays and deaths, according to CDC data.
“The fact that communities with the greatest health disparities in this country are also the least represented in these studies is a critical part,” said Dr. Michele Andrasik, a Fred Hutch scientist who worked on the study. “Ensuring inclusion is really important.”
Researchers from Fred Hutch, Harvard, Emory University, and others studied 230 vaccine trials in the United States from 2011 to 2020. The trials included vaccines from flu to shingles and included 219,555 participants. They compared the number of people in each race and ethnic group to US census data from 2011 and 2018.
White people were over-represented, while Black / African American, Hispanic, and Native American / Alaskan Native Americans were under-represented, as were older adults. The Asian and Native Hawaiian / Pacific Islander participants were more closely aligned with the percentages of the total population and women were overrepresented.
About 10% of the vaccine study participants in the study data were Black / African American – data of about 13%. The number of Hispanic Americans was 11.6%, while that population was around 16-19%, according to the census data for the same period. Meanwhile, white Americans made up 78% of the study participants, compared to 74 to 76% in the censuses over the same period.
 Dr. Michele Andrasik, a scientist working for Fred Hutch, and Dr. Steven Pergam, a doctor from Fred Hutch. (Fred Hutch Photos)
Dr. Michele Andrasik, a scientist working for Fred Hutch, and Dr. Steven Pergam, a doctor from Fred Hutch. (Fred Hutch Photos)
There were some limitations. Although age and gender were reported in each study, only 58.3% included race and 34.3% included ethnicity.
This lack of information is noteworthy as the Food and Drug Administration recommends such data collection, said Dr. Steven Pergam, a Fred Hutch doctor specializing in the prevention and treatment of infectious diseases. In addition, data gaps have impacted the nationwide spread of COVID-19 vaccines, he said. It is possible that participants may not answer questions about race and ethnicity, or that study interviewers may not be able to visually identify their own race and ethnicity.
“Some people feel uncomfortable asking people about their race and ethnicity, so discomfort is an obstacle,” Andrasik said.
Can vaccinated people still transmit COVID-19? The answer is the key to herd immunity
The researchers recommended that racial and ethnic data collection be enforced and that population-reflective diversity goals be included in all vaccination attempts with major infections. They also urged that enrollment target populations at greatest risk of infection or mortality, such as older adults with the COVID-19 pandemic.
Creating vaccine trials that reflect the whole community can have far-reaching implications, such as: B. Addressing vaccination hesitation and safety concerns.
“Because of past exclusion and abuse, vaccine reluctance and lack of trust in the medical facility may be more common in minority groups, making inclusion even more important,” the study authors write.
At the beginning of the process, steps can be taken that prioritize inclusion – a goal of the COVID vaccine trials. This engagement takes place prior to the start of court hearings, with active participation in community and relationship building.
According to the study, underrepresentation may be most evident in the later stages of vaccine development. Cost combined with quick enrollment goals can be a reason.
According to researchers, it is important to set specific racial and ethnicity goals for participants that reflect the population.
“When you know who to hire it’s amazing how it will happen,” said Andrasik. “If you are vigilant and involve the community early and often, this can happen.”
Andrasik realized the importance of being included early on in their own experience with the COVID-19 vaccine. She had many conversations with black relatives and friends, many of whom expressed her discomfort and fear of vaccinations. In long conversations, she shared information about the inclusion efforts and the different populations involved in the COVID-19 studies.
“Then I have pictures on my phone showing that they were vaccinated,” she said. “It was really wonderful.”